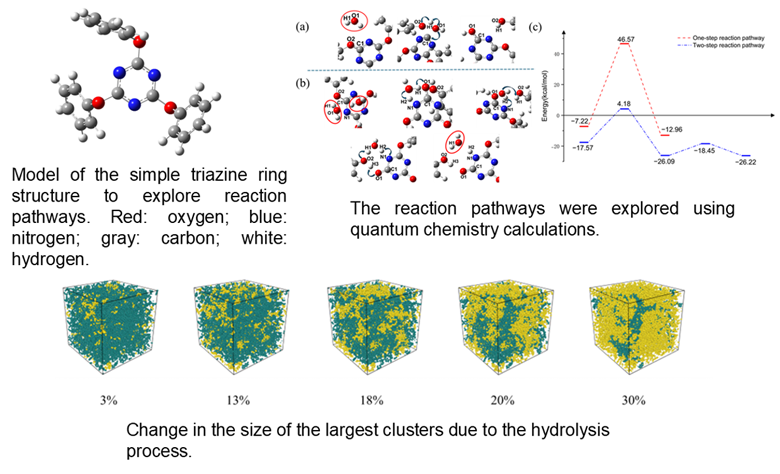
Polymer hydrolysis, where water breaks bonds in polymer chains, is experimentally challenging to observe directly. To overcome this, our work combines quantum-chemistry (QC) calculations with molecular-dynamics (MD) simulations to track cyanate-ester resin degradation from initial water uptake to final bond breaking, analyzing microstructural and physicochemical changes.
QC calculations on a simple triazine model revealed two hydrolysis pathways: a single-water, one-step process and a two-water, two-step process, with the latter significantly favored by hydrogen-bond assistance. We incorporated this energetically preferred two-water mechanism into MD simulations of a fully cured resin network via a Python-based reaction model that automatically breaks bonds when QC-derived geometric and energetic criteria are met.
Under humid conditions, our simulations accurately matched experimental trends in glass-transition temperature (Tg) changes, which we analyzed through cluster size and polymer chain motion. This framework precisely captures water-driven aging at a molecular level, offering practical insights for designing next-generation, humidity-resistant polymer materials.
Reference: Y. Bai, G. Kikugawa, and N. Kishimoto, to be submitted.
