The tissues that consist of our bodies are formed by highly organized cell populations, and the individual cells maintain the life of the organism by sensing and responding to various stimuli from the external environments. Mechanical force is one of the stimuli and the mechanical stress response is a cellular response in which cells sense mechanical forces through proteins that act as mechanosensors linked to cell membrane and cytoskeletons. The actin cytoskeleton is a dynamic intracellular structure that regulates cell motility and morphology, and is important for mechanical stress responses by these properties. Actin cytoskeletal remodeling is regulated by Rho family small GTPases, and the Rho GTPases are activated by RhoGEFs, which are composed of diverse proteins of the Dbl and Dock families. We focused on RhoGEFs to understand the mechanisms of actin cytoskeletal remodeling in mechanical stress responses. We previously revealed that Solo, a RhoA-targeting GEF is required for tensile force-induced RhoA activation and subsequent actin cytoskeletal remodeling. However, the molecular mechanism of Solo-mediated mechanical stress response is poorly understood. We searched for Solo-interacting proteins by proteomic analysis using proximity-dependent biotin identification (BioID) method and identified PDZ-RhoGEF (PRG) as a candidate of Solo-interacting protein. While PRG was localized at the cell peripheral area and induced lamellipodia formation, when PRG was co-expressed with Solo, PRG translocated to the Solo accumulation sites at the basal plane of the cell and enhanced Solo-induced actin polymerization (Fig. 1). Furthermore, we examined the effects of Solo-PRG interaction on their GEF activity using an in vitro exchange assay and revealed that Solo directly activates the GEF activity of PRG. We also showed that the interaction between Solo and PRG is required for the actin stress fiber formation in response to substrate stiffness. These results indicate that Solo-PRG, a novel RhoGEF signaling cascade, plays a role in actin cytoskeletal remodeling in response to substrate stiffness (Fig. 2).
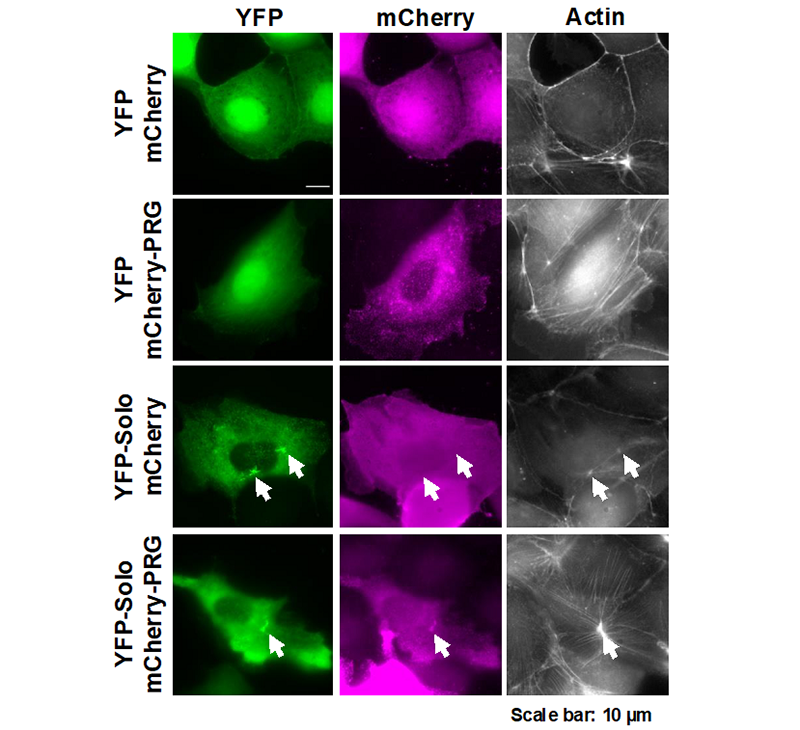
Figure 1. MDCK cells were transfected with YFP-Solo (green) and mcherry-PRG (magenta) alone or in combination, and the effect on the subcellular localization of each and the shape of the actin cytoskeleton was observed. (in the case of transfection with only YFP-Solo and mcherry-PRG, the another fluorescent protein fused to other protein of interest was co-transfected.) The arrows indicate the sites where YFP-Solo accumulates in the cells.
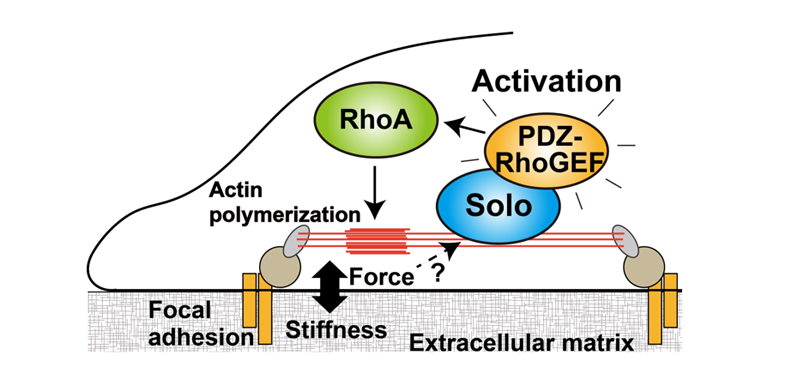
Figure 2. A model of the molecular mechanism by which cells regulate actin cytoskeleton remodeling in response to substrate stiffness through Solo and PDZ-RhoGEF.
