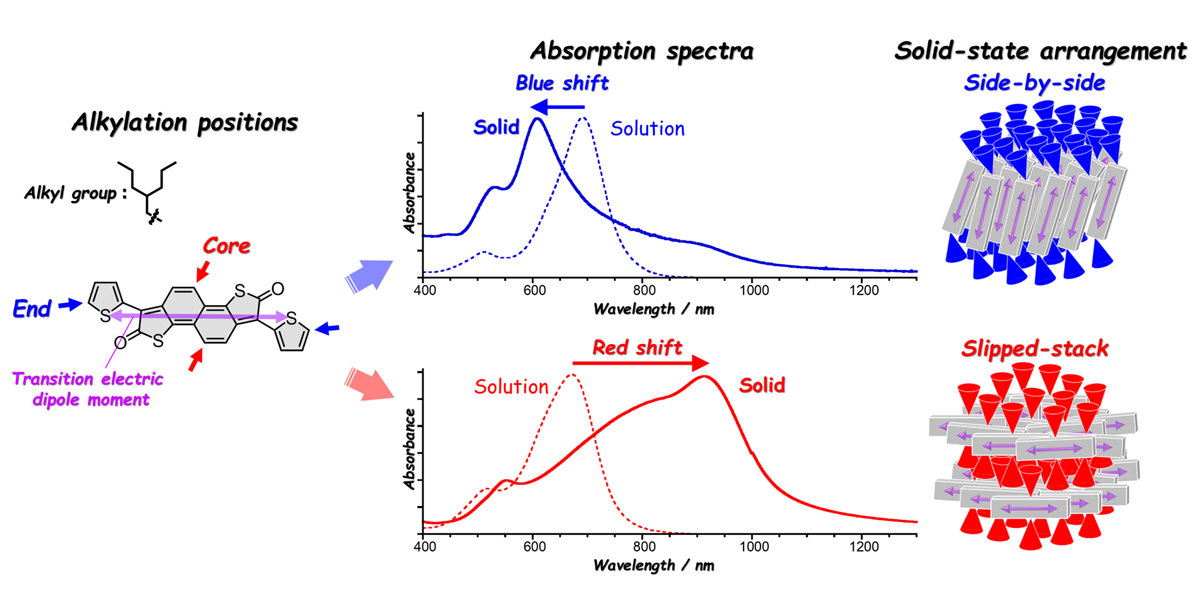
Organic semiconductors are attracting attention as next-generation materials for printed electronics. Carrier transport and light absorption/emission properties of organic semiconductors are provided by the π-conjugated skeleton (alternating single and double bonds) shown in gray in the figure. However, it is known that even with the same π-conjugated structure, their semiconducting properties vary greatly depending on their arrangement in the solid state. Therefore, controlling the molecular arrangement in the solid state is important but not easy. This study focused on gaining insight into controlling molecular arrangements and thus properties. We systematically synthesized new molecules with alkyl groups at different positions (end or core positions) on a near-infrared absorbing π-conjugated skeleton to investigate the relationship between the chemical structure, solid-state structure, and semiconducting properties of the molecules. While absorption wavelengths were similar in solution, solid-state optical absorption properties varied significantly depending on alkyl group positions. Namely, end-alkylation caused blue shifts, while core-alkylation led to red shifts. We found that the end-alkylation promoted side-by-side arrangements of π-conjugated skeleton, whereas core-alkylation caused slipped-stacking along the longitudinal axis, the differences of which altered the light absorption properties. Additionally, core-alkylation improved solubility in organic solvents by preventing π-conjugated structures from strong intermolecular interaction, which is crucial for solution processing in the fabrication of thin films. This study demonstrates that strategic molecular design can effectively control solid-state molecular arrangements, providing valuable insights into molecular design for tailoring properties of organic semiconductors.