Infrared spectroscopy of protonated dimethylamine clusters, H+(DMA)n, (n = 3–7), and their Ar-tagged clusters was performed to explore their hydrogen bond network structures. A stable isomer search and vibrational spectral simulations of the clusters were also carried out to support the interpretations of the observed spectra. Weakly hydrogen-bonded NH stretching vibrational bands, which are characteristic of the double acceptor site in cyclic structures of small-sized protonated clusters, are observed in the spectra of the Ar-tagged clusters of n ≥ 5, while only linear chain type structures are suggested for the Ar-tagged clusters of n = 3–4 and the bare clusters of all the sizes. These results demonstrate that the size and temperature dependence of the hydrogen bond network structures of the protonated dimethylamine clusters is analogous to that of protonated monohydric alcohol clusters.
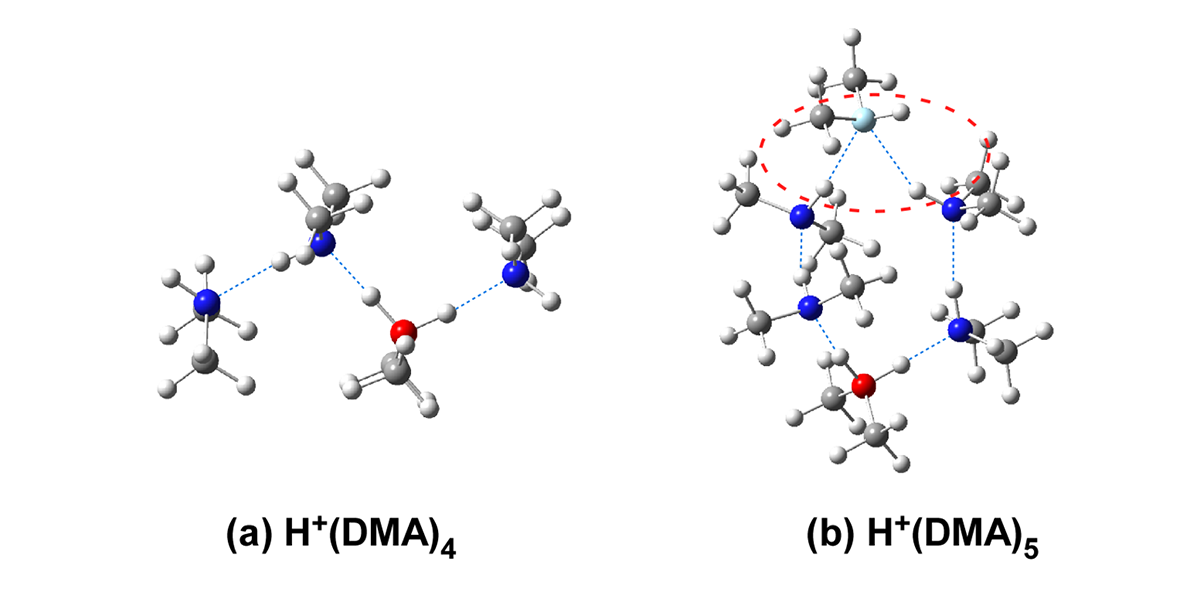
Fig. 1 Hydrogen bond structures of H+(DMA)n (a) n = 4 of the linear type network. (b) n = 5 of the cyclic type network with the double acceptor site formation (red dotted circle).
