Dihydrogen molecule and benzene are important energy resources, and their activation leading to useful chemicals is one of ongoing topics in the synthetic chemistry. The development of new activation methods of these molecules using ubiquitous metals and elements rather than expensive noble metals is highly required from the points of energy problems and environmental issues.
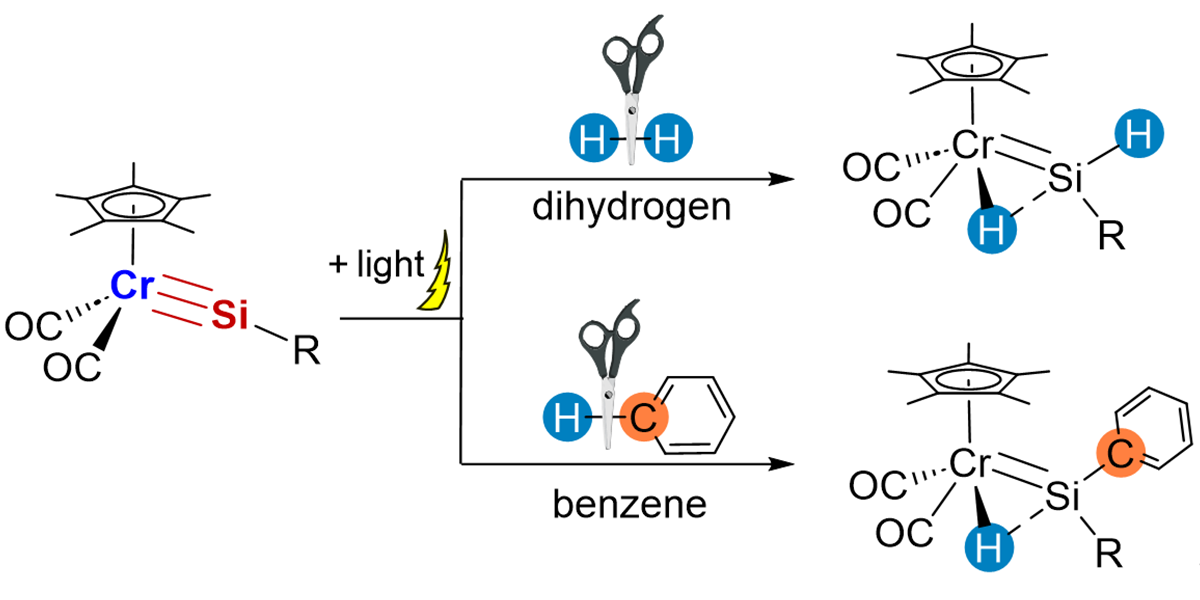
As ubiquitous elements, our group recently focused on chromium and silicon, and synthesized a silylyne complex featuring a Cr≡Si triple bond. Its strong polarity at the Cr(δ-)-Si(δ+) triple bond was revealed experimentally and theoretically. The chromium complex easily activated H2 under light (365 nm) irradiation to produce a silylene complex with a (H)Cr=Si(H) moiety, through 1,2-H–H addition across the Cr≡Si triple bond. Similarly, the chromium complex reacted with benzene under irradiation to afford an 1,2-addition product, via benzene C–H bond activation accompanied by Si–C bond forming. These results demonstrate a new class of metal–ligand cooperative activation, which can be applied to new synthetic approaches to useful materials.