One of the goals of molecular science is to be able to freely transform (edit) the structure of a molecule. Normally, transforming the structure of a molecule requires repeating chemical reactions with breaking and making chemical bonds. During these processes, undesired chemical reactions often occur, such as transforming the structure of parts of the molecule that you don't want to edit. Therefore, it is difficult to edit the structure of molecules as desired.
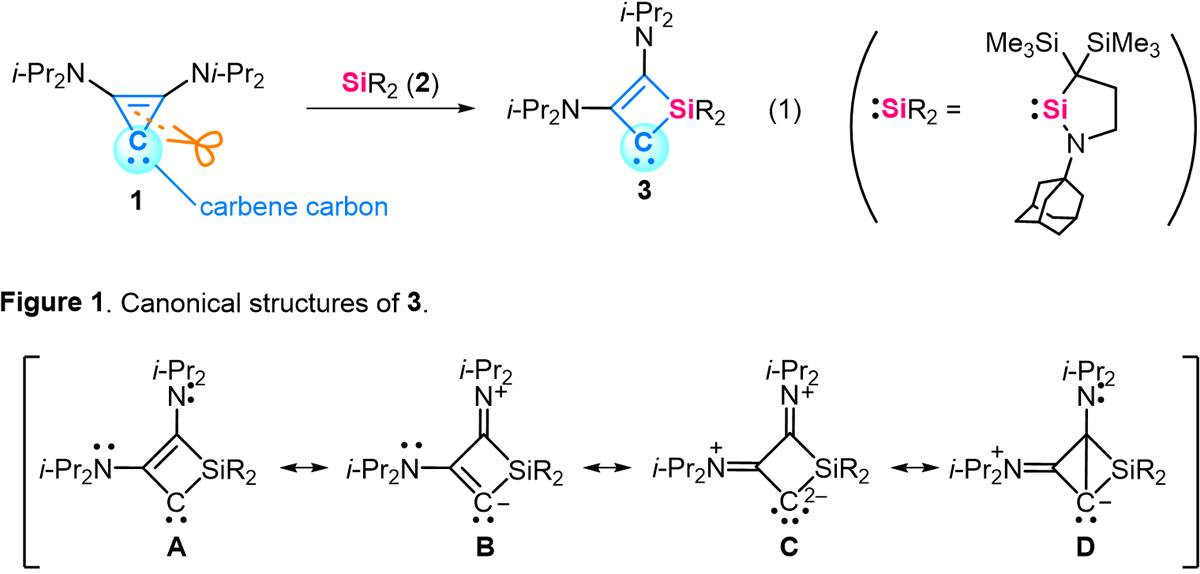
We discovered a reaction that inserts silicon units into the ring skeletons of highly reactive organic molecules (equation 1). In this reaction, compound 1 (a compound with a two-coordinate carbon atom, called a carbene) reacts with an organosilicon compound 2 to produce carbene 3. Although carbenes have a highly reactive two-coordinate carbon center, in this reaction, the two-coordinate carbon center is formally preserved. In addition, carbene 3 is an unprecedented carbene with a four-membered ring structure, a carbon-carbon double bond, and a two-coordinate carbon. Carbene 3 is found to have the properties of various structural formulas (Figure 1) and reacts with inert molecules such as carbon monoxide and carbon dioxide.