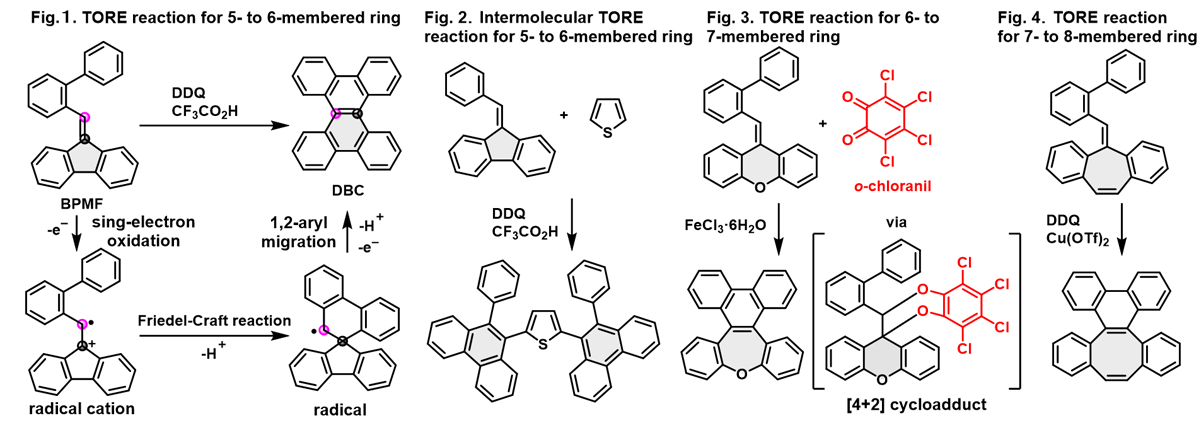
The impressive structural diversity of polycyclic aromatic hydrocarbons (PAHs) and their heteroaromatic analogues, consisting of pentagonal, hexagonal, heptagonal, and octagonal rings has continued to drum up interest in the fields of synthetic chemistry and materials science owing to their tunable electronic properties in relation to their unique planar, twisted, and curved π-surface conformations. Although an array of synthetic strategies have been developed over the past decades to construct both known and novel PAH scaffolds, flexible and expedient strategies to create PAHs with embedded rings of different sizes are rare. In 2017, we designed and developed a novel and highly efficient tandem oxidative ring expansion (TORE) reaction, which was proposed to undergo single-electron oxidation of the alkene moiety of the BPMF molecule, followed by Friedel-Crafts spirocyclization and 1,2-aryl migration to produce the desired dibenzo[g,p]chrysene (DBC) scaffold (Fig. 1, Ref. 1). Later, this TORE strategy was successfully extended to the construction of structurally diverse PAHs involving hexagon-embedded 9,10-diaryl phenanthrenes, O- or S-incorporated heptagon-embedded PAHs with negative curvature (Ref. 2), and octagon-embedded PAHs with saddle-shaped conformation (Figs. 2-4). We expect that our TORE strategy will accelerate research in synthetic chemistry and electronic materials through the synthesis of highly π-extended new PAHs with diverse functionality.