In chemical bonds, electrons act like glue to bind nuclei together. An elementary particle “muon” (µ) has a mass 207 times greater than that of the electron and can bind two hydrogen nuclei more strongly than an electron can. We are studying muon-catalyzed fusion, in which nuclear fusion reaction occurs within a muonic molecule because of the strong chemical bonding by the muon.
The muon forms a very small 'muonic hydrogen atom' that is one 200th the size of a normal hydrogen atom. Figure 1 illustrates the size difference between a hydrogen atom and a muonic hydrogen atom.
When a muon is injected into a deuterium target, a muonic deuterium atom (dµ) is formed. Upon further collision with another d, a “muonic molecule” is formed (Fig. 2). Here, d represents the nucleus of deuterium. As the muonic molecule is approximately 100 times smaller than a hydrogen molecule, the two nuclei in the molecule can approach each other closely due to the tunneling effect, resulting in an intramolecular nuclear fusion reaction. After nuclear fusion, the muon is freed and can form another muonic molecule, causing another nuclear fusion. The muon itself is not consumed in the fusion reaction but repeatedly assists the reaction. Hence this series of reactions is called muon 'catalyzed' fusion (µCF).
Until now, the muons freed after the fusion in µCF (called 'recycling muons') have never been extracted and observed. We have studied these recycling muons both theoretically and experimentally.
First, we performed precise quantum mechanical calculations to predict the probability of the recycling muon being released and its kinetic energy distribution. The kinetic energy of recycling muons was found to peak at 1 keV.
In collaboration with the High Energy Accelerator Research Organization (KEK), we attempted to extract recycling muons into the vacuum using solid hydrogen. We developed a method to induce muon deuterium atoms into a thin film of solid deuterium to cause µCF, and to electrostatically extract and detect recycling muons emitted from the film into a vacuum (Fig. 3). 1 keV recycling muons were electrostatically transported for 1 meter and were stopped on a silver foil. The muons then emitted characteristic X-rays of muonic silver atoms. As shown in Figure 4, the recycling muons were successfully extracted for the first time.
Muons provided from accelerators has a high kinetic energy of about 4000 keV, but the energy is too high for various fields of application, and there was no way to reduce it. The recycling muons with energy of 1 keV can now be extracted in our experiments. This technology is expected to find applications in various fields, such as muon microscopy, three-dimensional non-destructive elemental analysis, and muon colliders.
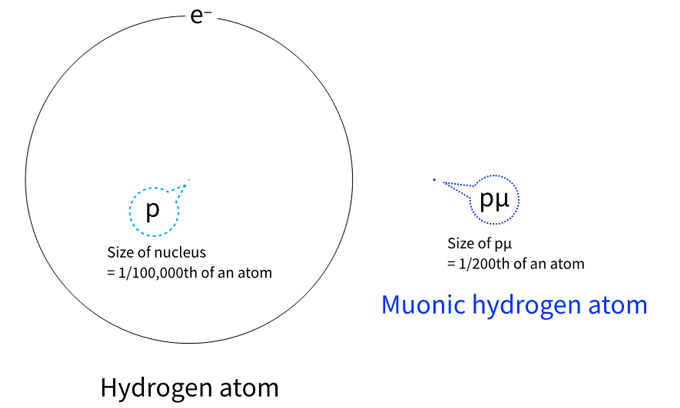
Figure 1 Size difference between a hydrogen atom and a muonic hydrogen atom
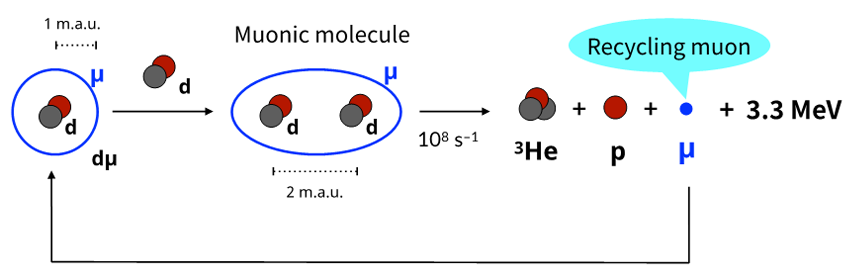
Figure 2 Reaction scheme of muon catalyzed fusion cycle
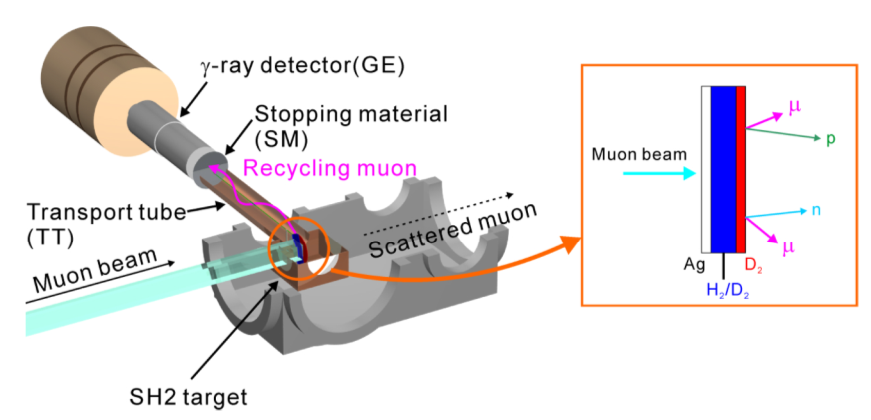
Figure 3 Illustration of apparatus to extract and detect recycling muons.
Reprinted from Fusion Engineering and Design Vol. 170, K. Okustu et al., “Design for detecting recycling muon after muon-catalyzed fusion reaction in solid hydrogen isotope target”, Page. 112712 (2021), with permission from Elsevier.
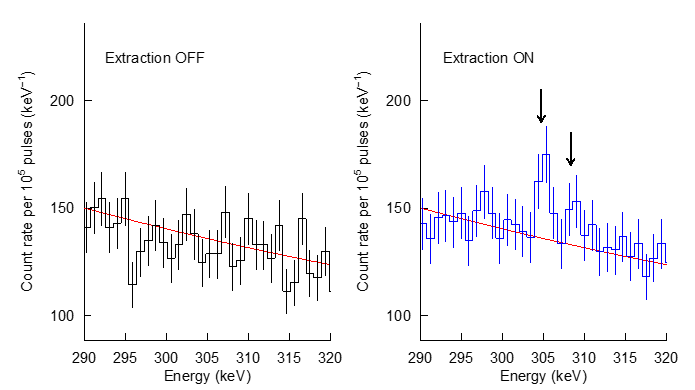
Figure 4 Changes of X-ray spectrum when the extraction voltage was applied.
