Organocatalysts are easy to handle because they are stable in water and air, and they have relatively low toxicity and low environmental impact. Thus, the field of organocatalysis has developed rapidly. We have developed diphenylprolinol silyl ether as an effective organocatalyst, which can be applied to many kinds of asymmetric reactions.
We propose the importance of “pot economy” because one-pot operations are efficient methods for making several bonds and can generate complex molecules in a single reaction vessel with several sequential reactions. Moreover, one-pot operations circumvent purification steps via in situ quenching, thereby minimizing chemical waste and saving time. Based on this concept, our group has investigated the synthesis of drugs and natural products in a small number of pots.
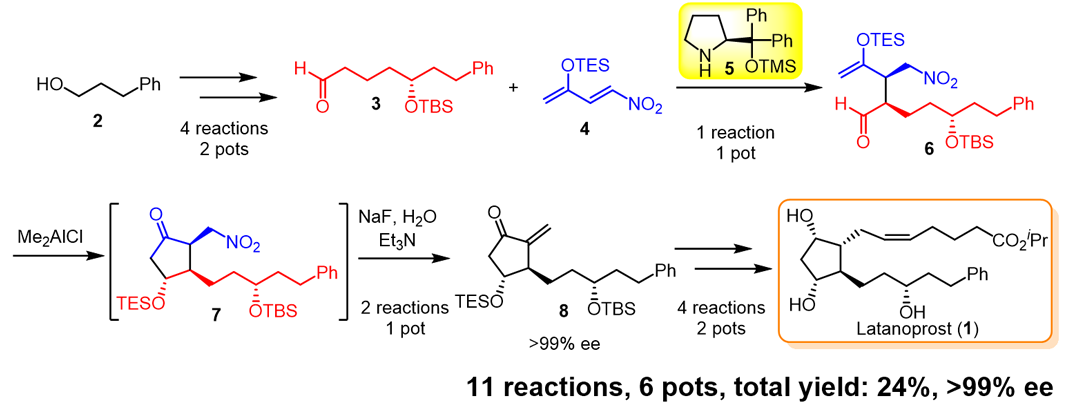
Latanoprost (1) is an antiglaucoma agent and an analog of the prostaglandin PGF2α. As it is a blockbuster drug developed by Pharmacia, it is one of the important targets in the synthesis of prostaglandins.
Recently, the enantioselective total synthesis of latanoprost (1) has been accomplished with excellent diastereo- and enantioselectivities in a pot-economical manner using six reaction vessels with a total yield of 24%. A key substituted cyclopentanone was synthesized by an organocatalyst-mediated Michael reaction and a substrate-controlled intramolecular Mukaiyama aldol reaction.