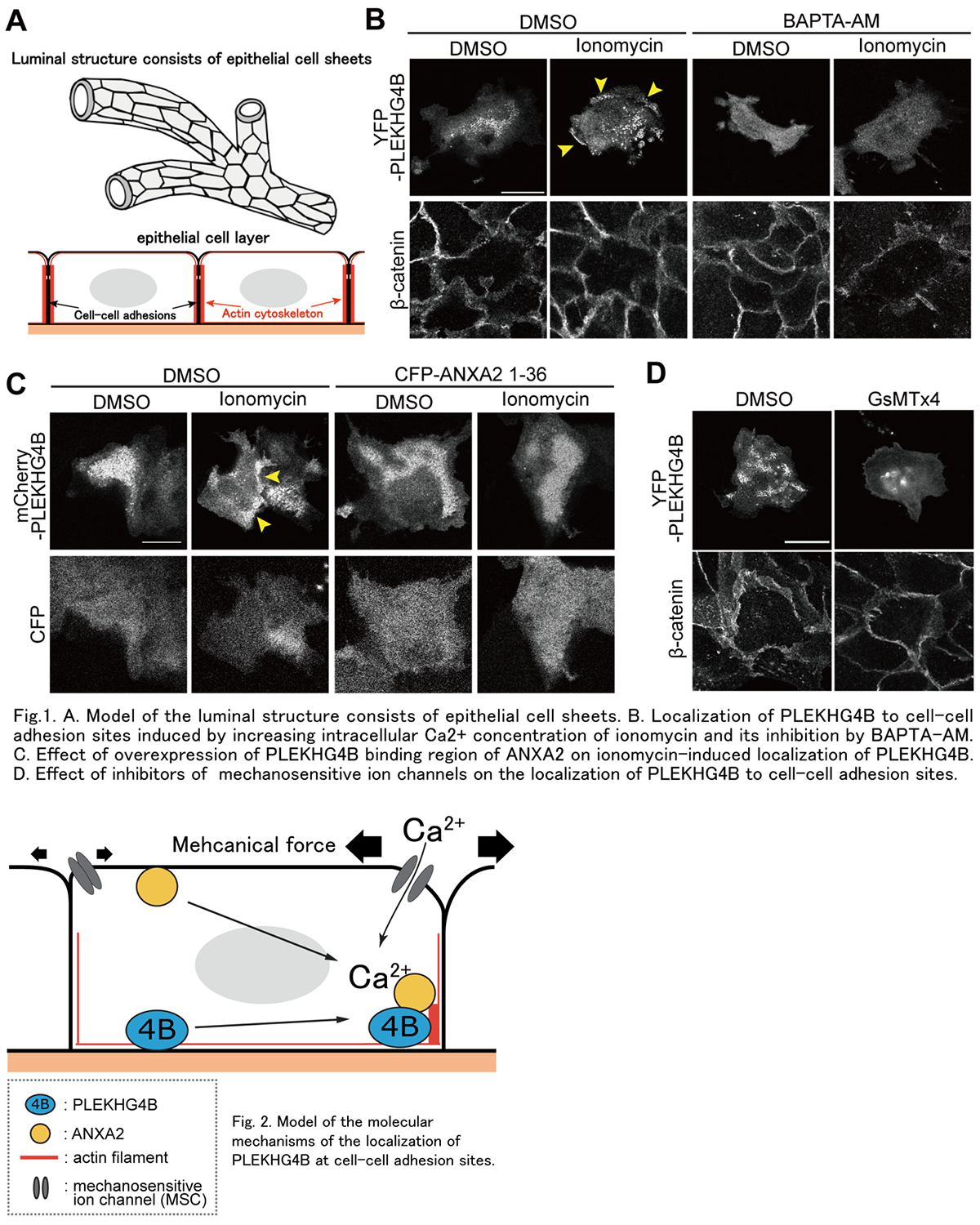
The organs in our body, including lung, kidney, liver, and various glands are composed of luminal structures formed by epithelial cell sheets. To form proper cell sheets, after cells contact each other, the cell-cell adhesion structure is formed by linking the cytoplasmic region of adhesion molecules with actin cytoskeleton as a supporting backbone (Fig. 1A). And cells sense the behavior of neighboring cells and spatiotemporally reorganize the actin cytoskeleton to adapt the changing situation. We previously found that PLEKHG4B, a regulatory protein of actin cytoskeleton reorganization as an activator of Cdc42, a Rho family small G protein, is required for the maturation of cell-cell adhesion of epithelial cells. In the present study, we aimed to elucidate the molecular mechanism of PLEKHG4B in the formation of cell-cell adhesion. Since we also found that PLEKHG4B associates with Annexin-A2 (ANXA2), a Ca2+-binding protein located on the plasma membrane, we analyzed the function of Ca2+ and ANXA2 on PLEKHG4B. Increasing the intracellular Ca2+ concentration by ionomycin induced the accumulation of PLEKHG4B at the basal side of cell-cell adhesion sites, whereas decreasing the Ca2+ concentration by BAPTA-AM (an intracellular Ca2+ chelator) suppressed the localization of PLEKHG4B at the basal membrane (Fig. 1B). Meanwhile, overexpression of the N-terminal region of ANXA2, which is a binding region of PLEKHG4B, suppressed the ionomycin-induced accumulation of PLEKHG4B at cell-cell adhesion sites (Fig. 1C). Then, the binding between PLEKHG4B and ANXA2 was increased by ionomycin treatment. Furthermore, inhibition of mechanosensitive ion channels (MSCs) suppressed PLEKHG4B localization at cell-cell adhesion sites (Fig. 1D). These results suggest that PLEKHG4B localizes to cell-cell adhesion sites in a Ca2+-bound ANXA2-dependent manner and that regulation of PLEKHG4 localization induces reorganization of the actin cytoskeleton at cell-cell adhesion sites to provide a proper mechanical environment there (Fig. 2).