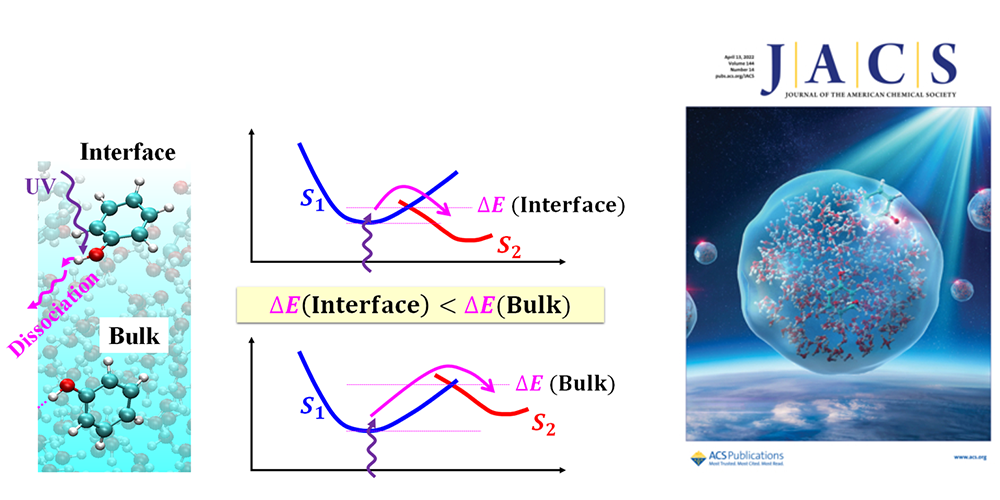
Photochemical reactions at the air–water interface can show remarkably different rates from those in bulk water. The present study elucidates the reaction mechanism of phenol characteristic at the air–water interface by the combination of molecular dynamics simulation and quantum chemical calculations of the excited states. We found that incomplete hydrogen bonding to phenol at the air–water interface affects excited states associated with the conical intersection and significantly reduces the reaction barrier, resulting in the distinctively facilitated rate in comparison with the bulk phase. The present study indicates that the reaction dynamics can be substantially different at the interfaces in general, reflecting the difference in the stabilization energy of the electronic states in markedly different solvation at the interface.