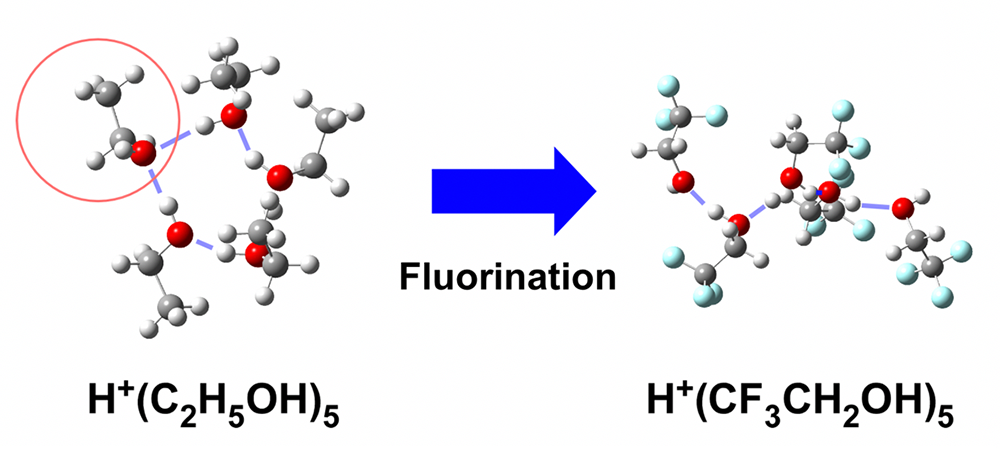
While protonated alcohol pentamers generally prefer to form cyclic hydrogen bond network structures, we found that the protonated pentamer of 2,2,2-trifluoroethanol never forms such cyclic hydrogen bond networks. It is interpreted that the stability of the double acceptor site is the key to determine the preference of hydrogen bond network structures in protonated alcohol clusters. This is an example of drastic intermolecular structural changes induced by substituent effects.