The carrier mobility of organic semiconductors in the solid state (crystallin state) depends greatly on the properties of individual molecules (molecular properties) and the arrangement of molecules in a crystal (crystal structure). While quantum chemical calculations have made it possible to predict molecular properties and develop a wide variety of organic semiconductor molecules using state-of-the-art organic synthesis, it is currently difficult to predict and control the crystal structure of organic semiconductors. It is important to control the crystal structure of organic semiconductors in order to achieve high carrier mobility, and this is recognized as one of the most challenging issues in the field of organic semiconductors.
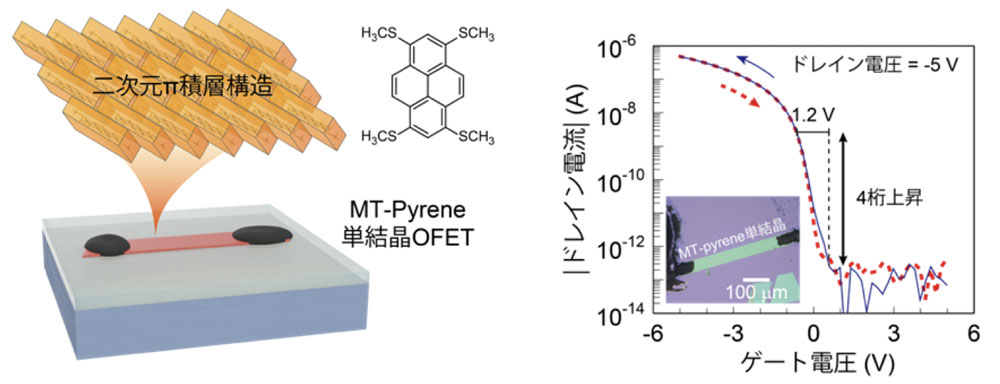
We have found that the selective introduction of a methylthio group (-SCH3), which is a simple substituent, at specific positions in the molecule is useful for controlling the crystal structure. In the present work, we focused on pyrene, a peri-condensed polycyclic aromatic hydrocarbon, and investigated its crystal structure and semiconductor properties. As a result, we found that MT-pyrene not only forms a new type of two-dimensional π-stacking structure but also exhibits very high carrier mobility of over 30 cm2 / Vs, as evaluated by field-effect transistor devices. Furthermore, the field-effect transistor of MT-pyrene can be driven at a low voltage of a few volts, and the temperature dependence of the carrier mobility reveals the band conduction, confirming that MT-pyrene is an excellent organic semiconductor. The results also strongly imply that the high carrier mobility of organic semiconductors can be achieved by controlling the crystal structure.