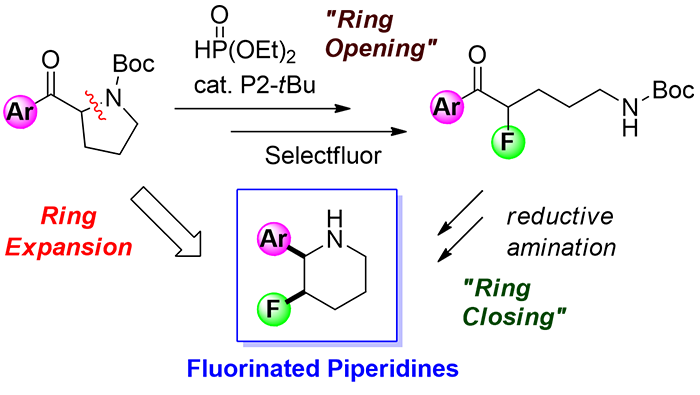
A ring expansion of 2-benzoylpyrrolidines was developed for the synthesis of fluorinated piperidines. The methodology involves the formal fluorinative ring opening utilizing the [1,2]-phospha-Brook rearrangement and a subsequent intramolecular reductive amination. The operationally simple three-step protocol offers an efficient access to 2-aryl-3-fluoropiperidines, which are difficult to synthesize by using conventional methods. The methodology was further applied to the synthesis of other nitrogen heterocycles, such as azepanes and tetrahydroquinolines.