The acidity of CH bond is generally very low because of the similar electronegativity of carbon and hydrogen atoms. Therefore, proton transfer from saturated hydrocarbons is ordinarily negligible. Such a situation, however, largely changes upon ionization of the molecule. We have demonstrated that pentane radical cation acts as strong acid to water, by infrared spectroscopy of mass-selected cations. Significant enhancement of the CH acidity upon ionization has been found also in many other organic compounds.
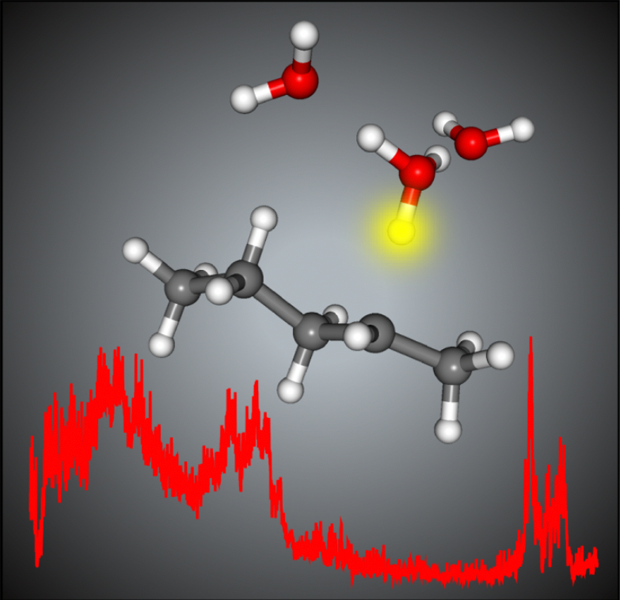
The most stable structure of the [pentane-(H2O)3]+ cation. A proton is transferred from the pentane moiety to the water moiety, and the hydronium ion is formed.
T. Endo, Y. Matsuda, A. Fujii,
Infrared spectroscopic study of the acidic CH bonds in hydrated clusters of cationic pentane.
J. Phys. Chem. Lett. 2017, 8, 4716-4719.
DOI: 10.1021/acs.jpclett.7b02282
https://pubs.acs.org/doi/10.1021/acs.jpclett.7b02282
Y. Matsuda, T. Endo, N. Mikami, A. Fujii, M. Morita, K. Takahashi,
The large variation in acidity of diethyl ether cation induced by internal rotation about a single covalent bond.
J. Phys. Chem. A 2015, 119, 4885-4890.
DOI: 10.1021/acs.jpca.5b02604
https://pubs.acs.org/doi/10.1021/acs.jpca.5b02604
M. Xie, Y. Matsuda, A. Fujii,
Infrared spectroscopic investigation of photoionization-induced acidic C-H bonds in cyclic ethers.
J. Phys. Chem. A 2015, 119, 5668−5675.
DOI: 10.1021/acs.jpca.5b03406
https://pubs.acs.org/doi/10.1021/acs.jpca.5b03406
M. Xie, Y. Matsuda, A. Fujii,
Infrared spectroscopic investigation of the acidic CH bonds in cationic n-alkanes: Pentane, hexane, and heptane.
J. Phys. Chem. A 2016, 120, 6351−6356.
DOI: 10.1021/acs.jpca.6b05567
https://pubs.acs.org/doi/10.1021/acs.jpca.6b05567
Y. Matsuda, M. Xie, A. Fujii,
An integrated experimental and theoretical reaction path search: Analyses of the multistage reaction of an ionized diethyl ether dimer involving isomerization, proton transfer, and dissociation.
Phys. Chem. Chem. Phys. 2018, 20, 14332-14338.
DOI: 10.1039/c7cp08566d
https://pubs.rsc.org/en/content/articlelanding/2018/cp/c7cp08566d#!divAbstract
T. Endo, Y. Matsuda, S. Moriyama, A. Fujii,
Infrared spectroscopic study on trimethyl amine radical cation: Correlation between proton-donating ability and structural deformation.
J. Phys. Chem. A 2019, 123, 5945-5950.
DOI: 10.1021/acs.jpca.9b01261
https://pubs.acs.org/doi/10.1021/acs.jpca.9b01261
