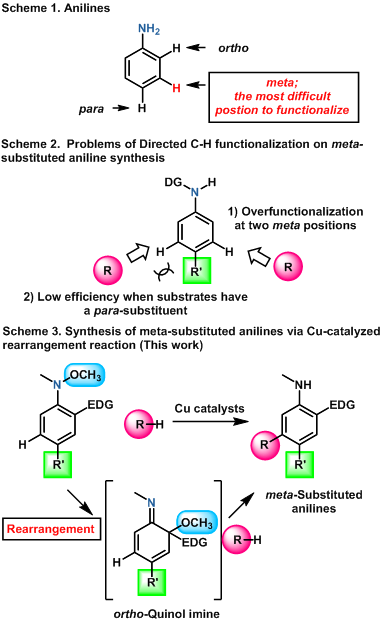
Aniline derivatives are widely utilized in various fields such as pharmaceuticals and organic devices. Therefore, it is of great importance to synthesize anilines having functional groups at proper positions in order to impart desired biological and physical properties. Since anilines inherently exhibit ortho- and para-preference in electrophilic substitution reactions, which have been generally utilized for functionalization of the aniline benzene ring, it is still challenging to develop efficient and selective methods to synthesize meta-substituted anilines (Scheme 1). Recently, transition metal-catalyzed C-H activation reactions have paid much attention for meta-functionalization using a directing group (DG) on the aniline nitrogen atom (Scheme 2). However, this state-of-the-art method still has some drawbacks, such as overfunctionalization at the two equally reactive meta-position and low reactivity of substrates having a substituent at the neighboring para position. In this context, we developed an entirely new approach to meta-substituted aniline derivatives based on a catalytic rearrangement reaction (Scheme 3). That is, we disclosed that the reactions between N-methoxyanilines having an electron-donating group, such as alkyl, 2-furyl, and methoxy groups, at the ortho-position, and carbon nucleophiles, such as 1,3,5-trimethoxybenzene, N-methylindole, and dimethyl malonate, were efficiently promoted by cationic copper catalysts ligated with an N-heterocyclic carbene ligand, affording the corresponding meta-substituted anilines in a selective manner. Because the key intermediate, ortho-quinol imine species, which undergoes addition of the carbon nucleophile, generates just one time in a single catalytic cycle, the present method is essentially free from overreaction. Moreover, a wide repertory of substituents, such as bromo, ester, vinyl, and methylthio groups, were tolerated as a para-substituent. We expect that meta-substituted anilines synthesized by our present method will be utilized for material development and drug discovery in future.
“Synthesis of meta-Substituted Anilines via Copper-Catalyzed [1,3]-Methoxy Rearrangement”
I. Nakamura, H. Tashiro, Y. Ishida, M. Terada, Org. Lett. Article ASAP.
https://pubs.acs.org/doi/10.1021/acs.orglett.0c01009
