Geometrical structures of cerium oxide cluster cations, CenOm+ (n = 2–6, m ≤ 2n), were studied by ion mobility mass spectrometry (IMMS). The most plausible structure for each composition was determined by comparison of a collision cross section (CCS) measured by IMMS with simulated CCSs of several candidate structures obtained from density functional theory (DFT) calculations. Structures with a peroxide ion (O22–) were found to be the most reasonable for CenO2n+ compositions because they are consistent with the experimental results and have the lowest energies among the examined candidate structures, whereas other structures without O22– are also possible. These findings offer important insights into the reaction mechanisms involving those clusters.
Reprinted with permission from J. Phys. Chem. C 2019, 123, 16641-16650. Copyright 2019 American Chemical Society.
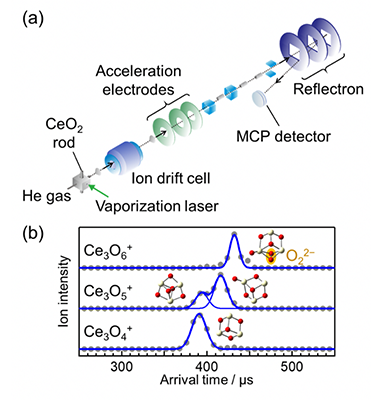
(a) Schematic view of the experimental apparatus for ion mobility mass spectrometry. In vacuum, cerium oxide cluster ions were firstly injected into the ion drift cell for ion mobility. After that the exited ions were analyzed in a time-of-flight mass spectrometer.
(b) Distributions of ion arrival time, which is almost proportional to the collision cross section, were plotted for the Ce3O4-6+ cluster ions. By comparison with the result of quantum chemical calculations, it is found that a peroxide O22- ion included in Ce3O6+.
T. Nagata, J. W. J. Wu, M. Nakano, K. Ohshimo, and F. Misaizu,
"Geometrical Structures of Gas Phase Cerium Oxide Cluster Cations Studied by Ion Mobility Mass Spectrometry,"
J. Phys. Chem. C 123, 16641-16650 (2019).
DOI: 10.1021/acs.jpcc.9b01378
https://pubs.acs.org/doi/10.1021/acs.jpcc.9b01378
