Our research is focusing on the design and development of novel chiral organocatalysts and their application to novel asymmetric transformations. Recently, we developed chiral bis(guanidino)iminophosphorane as a superbly active chiral organosuperbase catalyst, and successfully applied the catalyst to several enantioselective addition reactions of less acidic pro-nucleophiles, which were difficult to achieve by using conventional catalysts, owing to its strong basicity (probably the strongest basicity among reported chiral organobase catalysts).
This time, we have developed a novel enantioselective formal [3+2] cycloaddition of epoxides under Brønsted base catalysis. Our chiral organosuperbase catalyst enabled the enantioselective reaction of β,γ-epoxysulfones with imines to provide enantioenriched 1,3-oxazolidines possessing two stereogenic centers including a quaternary one in a highly diastereo- and enantioselective manner.
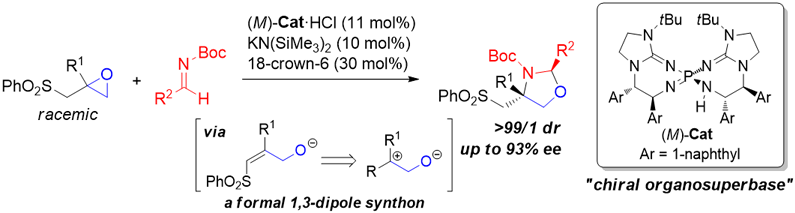
Enantioselective Formal [3+2] Cycloaddition of Epoxides with Imines under Brønsted Base Catalysis: Synthesis of 1,3‐Oxazolidines with Quaternary Stereogenic Center A. Kondoh, S. Akahira, M. Oishi, M. Terada,
Angew. Chem. Int. Ed. 2018, 57, 6299-6303. DOI: 10.1002/anie.201802468
