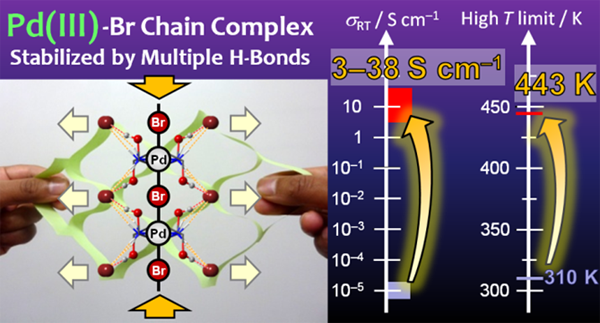
A Br-bridged Pd chain complex with the Pd ion in an uncommon +3 oxidation state, [Pd(dabdOH)2Br]Br2 (1), was prepared using a new method involving multiple hydrogen bonds. An in-plane ligand with an additional hydrogen donor group (hydroxy group) was used to create a multiple-hydrogen-bond network, which effectively shrinks the Pd−Br−Pd distance, stabilizing the Pd(III) state up to its decomposition temperature (443 K). 1 shows semiconducting behavior with quite high electrical conductivity (3−38 S cm−1 at room temperature), which is one million times larger than the previous record for analogous PdBr chains. Indeed, 1 is the most conductive MX-type chain complex reported so far. This new approach opens a new avenue of research toward the design of functional MX-type chain complexes in +3 oxidation state.
Multiple-Hydrogen-Bond Approach to Uncommon Pd(III) Oxidation State: A Pd−Br Chain with High Conductivity and Thermal Stability.
M. R. Mian, H. Iguchi, S. Takaishi, H. Murasugi, T. Miyamoto, H. Okamoto, H. Tanaka, S. Kuroda, B. K. Breedlove, M. Yamashita, J. Am. Chem. Soc. 2017, 139, 6562–6565.
DOI: 10.1021/jacs.7b02558
URL: http://pubs.acs.org/doi/10.1021/jacs.7b02558
