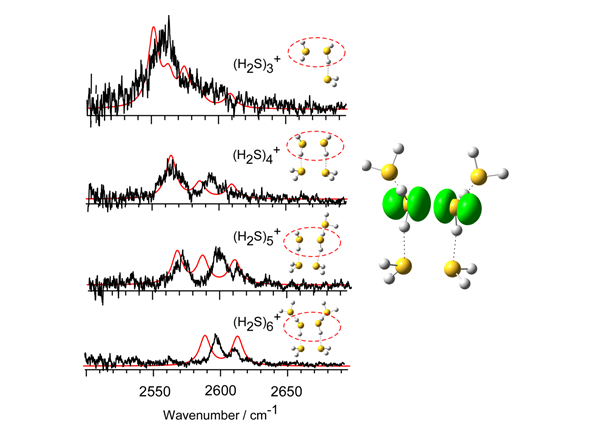
A covalent bond is formed by sharing two electrons by two atoms. But molecular orbital consideration suggests that an effective bond can be formed by sharing three electrons. Such a bond is called hemibond or two-center three-electron (2c–3e) bond. A hemibond is a non-classical chemical bond, and its existence has been supposed in radical cation clusters of molecules which have lone pairs. Though the nature of the hemibond and its role in the reactivity of radical cations have attracted great interest, spectroscopic observations of hemibonded structures have been very scarce. We have recently demonstrated the presence of a stable hemibonded core (H2S∴SH2)+ in the (H2S)n+ clusters (n = 3–6) in the gas phase by infrared spectroscopy combined with quantum chemical calculations. The spectral features of the free SH stretch of the ion core show that the hemibond motif of the ion core is maintained up to the completion of the first H-bonded solvation shell.
Dandan Wang, Asuka Fujii, Chem. Sci. 2017, 8, 2667-2670. DOI: 10.1039/c6sc05361k
http://pubs.rsc.org/en/Content/ArticleLanding/2017/SC/c6sc05361k#!divAbstract
