There are "anti-particles" such as positron (e+) and antiproton (P) with an opposite sign of charge for particles such as electrons and protons. In recent years, as the technology of creating and accumulating a large amount of antiparticles has become available, it has become possible to study the chemical reaction between antiparticles and atoms / molecules. We aim to reveal the change of chemical properties when positrons and antiprotons are bonded to atoms/molecules by using supercomputers.
The figure shows a negative copper ion (Cu-, which exists stably in vacuum) and a positronic copper atom (e+Cu), in which an electron or positron is bonded to a copper atom (Cu). In the positronic copper atom, the positron is distributed like an electron and forms a positron cloud around the copper atom, and then its diameter is about 3 times larger than the copper atom. In the case of a copper negative ion, since the electron enters the same orbital as the outermost electron in the copper atom, the change in size is small, in contrast to positron coupling. In other words, only the difference between the positron and electron charges changes the appearance of copper atoms. When a copper atom is replaced with a sodium atom (Na), the widely distributed positron forms a positronium (Ps) by extracting an electron in the sodium atom, and eventually a lager atom (NaPs+) is formed. The positronium consisting of an electron and a positron is an exotic atom (an atom containing other particles than a nucleus and an electron). The positronium has the same diameter as a hydrogen atom, but its mass is only one nine-hundredth of a hydrogen atom.
An "antihydrogen atom (H)" consisting of a positron and an antiproton is the smallest antimatter (matter composed only of antiparticles). We are interested in the chemical reactions of the antihydrogen atom; e.g., a reaction to produce a positive antihydrogen ion (H+) via positronium anti-hydride by a collision with a positronium,
H + Ps → HPs → H+ + e-
and a reaction to produce a positronium and protonium (Pn) via "hydrogenium (HH)" by collision with a hydrogen atom,
H + H → HH → Pn + Ps
These reactions play an important role in studying the grand question, "Why the universe consists of only matter not antimatter?". Since the positive antihydrogen ion can easily be controled in the electromagnetic field and an antihydrogen can be taken out at any time by photoionization, creation of this ion is the most important issue. In experiments using valuable anti-particles, theoretical studies are indispensable. The hydrogenium is the smallest complex of matter and antimatter. A protonium is an exotic atom consisting of a proton and an antiproton, and the diameter is only 1800 times smaller than the hydrogen atom. The effect that the motions of nuclei and electrons interact each other has recently been drawing attention. The hydrogenium is found to be a suitable compound for investigating this effect.
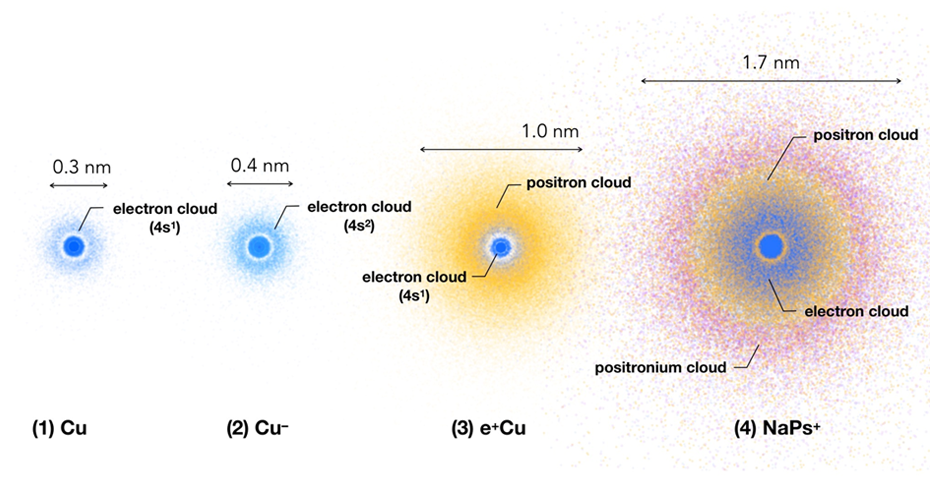
Figure: Distributions of outer-shell electrons, a positron and a postronium in (1) copper atom, (2) negative copper ion, (3) positron copper atom, and (4) positron sodium atom. The electrons are shown in blue, the positron in orange, and the positronium in purple. (1 nm is 10–9 m)
T. Yamashita, M. Umair and Y. Kino
J. Phys. B: At. Mol. Opt. Phys. 50, 205002 (2017).
DOI doi:10.1088/1361-6455/aa8b3b
URL http://iopscience.iop.org/article/10.1088/1361-6455/aa8b3b/meta
T. Yamashita, Y. Kino, E. Hiyama, S. Jonsell, P. Froelich
J. Phys.: Conf. Ser. 875, 052031 (2017).
DOI doi:10.1088/1742-6596/875/6/052031
URL http://iopscience.iop.org/article/10.1088/1742-6596/875/6/052031/meta
