We have been investigating the organocatalyst, which is composed by only C, N, O, H and so on without metal, because organocatalyst has several synthetic advantages; product is free from the contamination of metal, the catalyst is inexpensive, and the exclusion of water and air is not necessary. We have developed diphenylprolinol silyl ether as an effective organocatalyst, which can be applied to many kinds of reactions.
We have been applying this catalyst to the synthesis of (-)-oseltamivir, a neuraminidase inhibitor, which is one of the most effective drugs for the treatment of influenza.
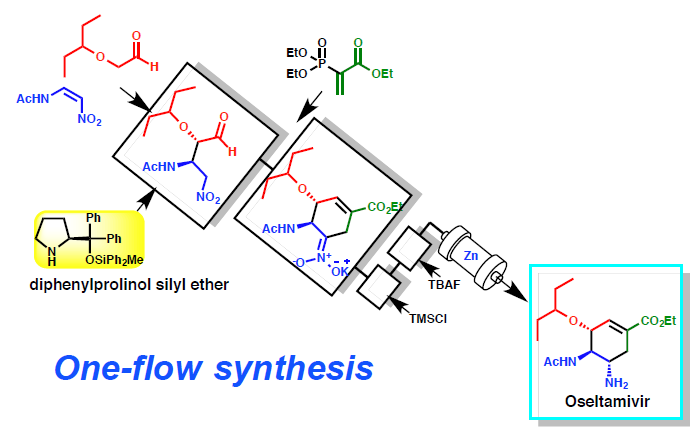
We already reported one-pot synthesis of (-)-oseltamivir using asymmetric reaction catalyzed by diphenylprolinol silyl ether as an organocatalyst.
On the other hand, a flow synthesis has attracted considerable attention recently because of its efficiency, productivity, safety, and reproducibility.
Recently a continuous flow synthesis of (–)-oseltamivir was accomplished, which is composed of five flow units. In each unit, the following reactions proceed efficiently: 1) a diphenylprolinol silyl ether mediated Michael reaction, 2) a domino reaction of Michael and intermolecular Horner–Wardsworth–Emmons reactions, 3) protonation, 4) epimerization, and 5) reduction of a nitro group to an amine. (–)-Oseltamivir was obtained in 13% total yield via a single flow with a residence time of 310 minutes.
S. Ogasawara, Y. Hayashi, Synthesis 2017, 49, 424-428.
DOI: 10.1055/s-2016-0036-1588899.
Invited paper of the special issue dedicated to the 70th birthday of Prof. D. Enders.
