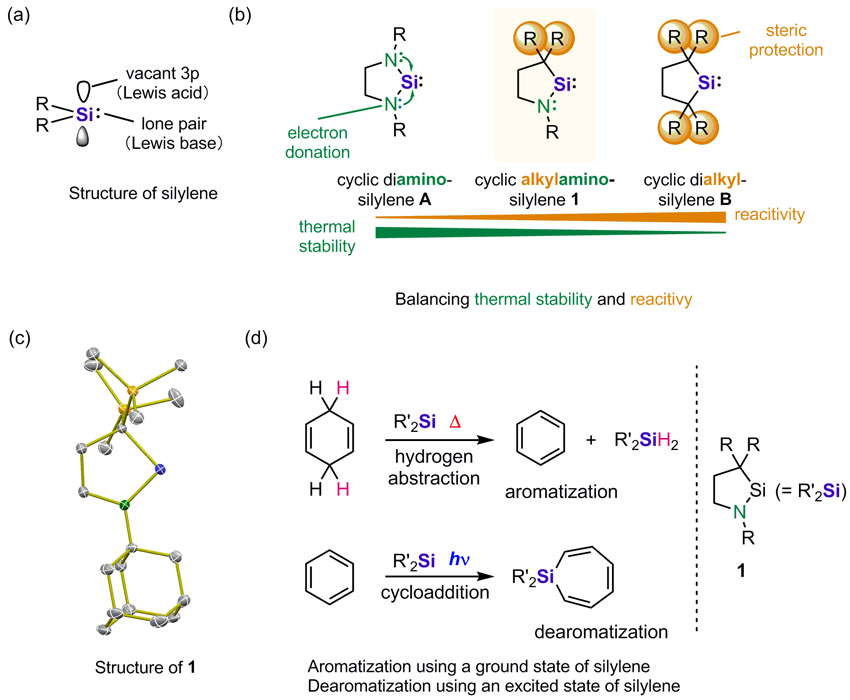
A crystalline two-coordinate cyclic (alkyl)(amino)silylene (1) was successfully synthesized and isolated. Its 29Si NMR and UV/Vis spectra indicate that the electronic properties of 1 fall between those of cyclic dialkylsilylenes and diaminosilylenes. At very low temperature, the color of a solution of 1 turned from colorless to yellow, which was monitored by UV/Vis spectroscopy. DFT calculations supported the hypothesis that head-to-head dimers (disilenes) with a very long Si–Si distance are formed at such low temperatures. Although 1 is thermally stable, it readily undergoes cycloadditions, Si−H insertions, and photochemical reactions with benzene similar to dialkylsilylenes. At higher temperatures, 1 is also susceptible to intermolecular benzylic C−H insertion reactions, as well as unprecedented dehydrogenation reactions with cyclohexa-1,4-diene and 9,10-dihydroanthracene to afford benzene and anthracene, respectively.
A Two-Coordinate Cyclic (Alkyl)(amino)silylene: Balancing Thermal Stability and Reactivity
Tomoyuki Kosai, Shintaro Ishida, Takeaki Iwamoto
Angew. Chem. Int. Ed. 2016, 50, 15554-15558
DOI: 10.1002/anie.201608736
URL: http://onlinelibrary.wiley.com/doi/10.1002/anie.201608736/abstract
